Syntheses of poly(styrene-acrylic
acid)iron complexes with 1,10- phenanthroline and their catalytic activities for
butadiene polymerization
Qu Yahuan,Wang Xiaoju and Chen Ruizhan
(Department of Chemistry,Changchun Normal Colleg)
聚(苯乙烯-丙烯酸)载体铁邻菲咯啉配合物的合成及其对丁二烯聚合的催化活性曲雅焕王晓菊陈瑞战(长春师范学院化学系,130032)合成了不同邻菲咯啉(Phen)含量的聚(苯乙烯-丙烯酸)载体铁邻菲咯啉配合物(SAAC.Fe.Phen)。SAAC.Fe.Phen-(i-Bu)3Al二元体系的催化效率是SAAC.Fe-Phen-(i-Bu)3Al三元体系的2倍,是小分子铁的400倍。当Phen/Fe(摩尔比)为0.7左右时,催化活性最高;催化活性随着Al/Fe(摩尔比)的增大而升高,但当Al/Fe大于70后活性趋于恒定。关键词:聚(苯乙烯-丙烯酸);铁邻菲咯啉配合物;丁二烯;聚合
In this paper, 1,10-phenanthroline(Phen) with poly(styrene-acrylic acid)
iron(SAAC· Fe) complexes(SAAC· Fe· Phen) have been synthesized and
their catalytic behaviors for butadiene polymerization are described.
Poly(styrene-acrylic acid )(SAAC)and polymer-supported Fe complexes (SAAC·
Fe) were prepared according to the method of reference[1].SAAC· Fe
· Phen was prepared by the following procedure: SAAC· Fe and Phen were mixed
in ethanol in the different mole ratio of Phen/Fe. The mixture was refluxed for 4 h and
then distilled off solvent. The reaction products were dried for 48 h under vacuum.
We synthesized a series complexes of SAAC· Fe· Phen by varying
Phen/Fe(mole ratio) and results showed that the Phen in complexes increases with
increasing Phen, but all the Phen/Fe were less than 1.In the IR spectra of SAAC· Fe·
Phen, the bands of C=N stretching vibration of Phen(1 561 cm-1) have
shifted to lower wavenumbers(1 535 cm-1).This suggested that Phen has been
coordinated to SAAC· Fe[2].
The catalytic system studied in this paper is the binary system of SAAC· Fe·
Phen- (i-Bu)3Al, its catalytic efficiency for butadiene polymerization is about twice
as much as that of SAAC· Fe· Phen-(i-Bu)3Al ternary system reported from
reference[1], and about 400 times that of the small molecule catalyst[3].
Fig 1 shows that the catalyticactivity of the system increases with increasing Phen /Fe.
When Phen /Fe is 0.7,there is a peak value, after that the activity decreases.
Fig 2 shows that the catalytic activity increases with increasing Al/Fe (mole
ratio) and the catalytic activity tends stability till Al/Fe value reaches above 70,the
intrinsic viscosity[η] of polymerization decreases with increasing the ratio of Al/Fe.
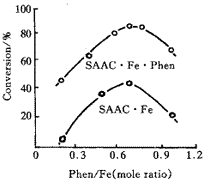
Fig 1 Effect of Phen/Fe on the activity
Polymerization conditions: Nd/Bd= 5× 10- 7 (mol/g),
Al/Fe (mole ratio)=70,20 ℃ ,10 min.
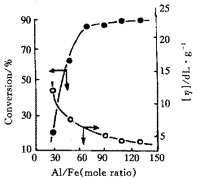
Fig2 Effect of Al/Fe on the activety
(Polymerization conditions same as in Fig 1.)
References
[1]Li Yuliang,Pang Shufen,Hang Dong,et al.Chin J Catal,1984,5(2):174
[2]Wang Liufang,Wu Jigui,Peng Zhouren,et al.Chin J Inorg Chim,1990,6(2):141
[3]Liu Guozhi,Wang Fengjiang.J Polym Comm,1985,4:252
Received:1999- 09- 10.
Biography:Qu Yahuan,associate professor. |